More Information
Submitted: April 21, 2023 | Approved: May 02, 2023 | Published: May 03, 2023
How to cite this article: Robinson JP. Patient’s perception of the benefits of long-term opioids: Reinforcement associated with short-term effects. J Addict Ther Res. 2023; 7: 008-011.
DOI: 10.29328/journal.jatr.1001026
Copyright License: © 2023 Robinson JP. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Patient’s perception of the benefits of long-term opioids: Reinforcement associated with short-term effects
James P Robinson*
Department of Physical Medicine and Rehabilitation, University of Washington, Seattle, WA, USA
*Address for Correspondence: James P Robinson, MD, PhD, Department of Physical Medicine and Rehabilitation, University of Washington, Seattle, WA, USA, Email: [email protected]
The appropriateness of opioid therapy for patients with Chronic Non-Malignant Pain (CNCP) has been questioned by a variety of researchers and policy makers [1-3]. One comprehensive review concluded that the benefits of such therapy for pain relief and functional improvement are modest at best and that opioid therapy is associated with significant harms including various symptoms (e.g., headache, nausea, sedation), development of opioid use disorder, fractures and death from overdose [3].
Surprisingly, research on the perceptions of opioids among CNCP patients has been sparse [3]. The research that is available suggests that in contrast to the skepticism about opioid therapy among some prescribers, researchers, legislators and regulators, many patients seem to be more positive about its benefits of it. For example, in a web-based survey of fibromyalgia patients, 75% of respondents who had been prescribed hydrocodone/acetaminophen reported that the medication was “helpful”, and 67% of those prescribed oxycodone/acetaminophen found that medication is helpful [4]. These ratings can be contrasted with clinical practice guidelines for fibromyalgia that recommend against the prescription of opioids [5]. One large-scale study involved telephone interviews of over 1,100 patients from a managed care facility [6]. The interviews included a 15-item Prescribed Opioid Difficulties Scale (PODS) to assess concerns that the patients had about opioid therapy. Consistent with the previously mentioned web-based survey of fibromyalgia patients, the investigators found that 61% of participants reported that opioids were “very helpful” or “extremely helpful” in relieving their pain. Similarly, Watson and colleagues [7] studied a cohort of 84 CNCP patients prescribed opioids and found that the patients generally indicated that their medications were effective, with the majority reporting that their opioids provided at least a 50% reduction in their pain. Patients reported few significant long-term adverse effects of opioid therapy. Robinson, et al. [8] found that patients on long-term opioid therapy gave generally positive global ratings of their medications, and were particularly emphatic in endorsing the idea that opioids gave them some control over their pain. Patients who were working tended to emphasize that their opioids permitted them to function better, whereas work-disabled patients were more likely to emphasize that their opioids facilitated changes in emotional functioning. Finally, qualitative studies have suggested that many CNCP patients report benefits from opioid therapy, although they often feel criticized by others because of the therapy [9-11]. The insistence by patients that they are deriving benefits from opioid therapy is frequently seen in clinical practice and creates stress for physicians as they try to negotiate the chasm that often exists between the beliefs and preferences of their patients and the expectations and demands from laws and regulations designed to curb opioid use [12-14].
One possible explanation for the apparent disconnect between the enthusiasm that many patients describe for their opioids and the unimpressive results of research on opioid therapy is that patients are rationalizing because they are addicted to their medications [15]. Although this is probably an adequate explanation for some patients, it almost certainly does not apply to all of them. Moreover, there is a danger that observers who attribute patients’ positive statements about opioids to addiction might fail to appreciate more persuasive reasons for the beliefs and preferences of the patients.
The general purpose of this essay is to promote the idea that the beliefs and values of patients receiving opioids for CNCP should be studied rather than ignored because they are attributed to addiction. The specific hypothesis outlined below is that many CNCP patients may value opioids because of short-term positive changes that they experience after taking doses of their medications. These changes might occur even if opioids do not lead to long-term changes in average pain levels or foster long-term benefits such as a return to work.
The proposed hypothesis is consistent with abundant research demonstrating that immediate reinforcement promotes the development of conditioned responses [16]. Moreover, there is evidence that when individuals are under stress, they are especially prone to placing high value on immediate rewards [17]. This has been demonstrated in patients with anxiety or depressive disorders [18,19], and in people subjected to economic stress [20] or experimentally induced social stress [21]. Thus, the general tendency for immediate reinforcers to be more potent than delayed ones is magnified among individuals who are experiencing various kinds of stress. Since pain is stressful, it is likely that CNCP patients will demonstrate preferences similar to that seen in other highly stressed groups.
In order to assess the validity of the hypothesis that CNCP patients value their opioids because of the short-term benefits they perceive after taking doses, it is necessary to assess these short-term effects and measure the value that patients place on their medications. Short-term changes in subjective state and neurophysiologic functioning following opioid doses have been studied among CNCP patients receiving opioid therapy [22-25] as well as among pain-free volunteers [26-29] and heroin addicts [30-33]. But these studies have not included assessments of the value that subjects placed on their opioids.
The hypothesis proposed here could be tested by having CNCP patients take doses of a short-acting opioid, assessing changes in their pain levels and stress levels over the hour following a dose and associating their responses with assessments of the value the patients place on opioids. The value assessments could be obtained by making use of well-established techniques that have been used extensively by behavioral economists. They have measured the value that people place on various commodities by determining the size of monetary rewards that individuals require in order to relinquish something (here, opioids), or the amount of money they are willing to pay in order to obtain something (here again, opioids) [34-38].
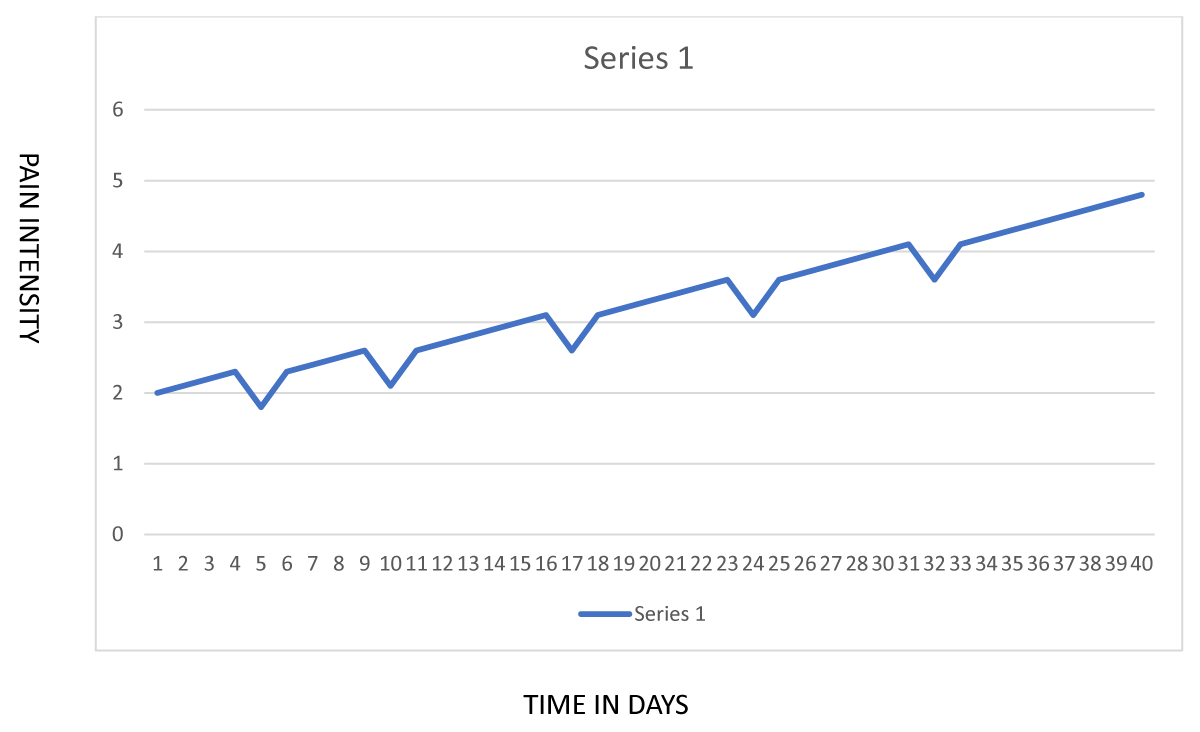
This hypothesis focuses attention on the ways in which opioids affect patients in the minutes to hours after a dose. It is entirely possible that patients experience relief from some combination of analgesia, sedation, and reduced emotional distress during the period when an opioid dose is acting even if they do not demonstrate cumulative improvements over weeks or months of opioid therapy. In fact, as shown in Figure 1, it is possible for a patient’s condition to deteriorate over time (perhaps because of opioid-induced hyperalgesia), but for the patient to be convinced of the benefits of opioids because of repeated episodes of short-term symptomatic relief.
Figure 1: Short term effects of opioid doses vs. long term changes in pain among CNCP patents.
This hypothesis highlights the possible mismatch between the long-term outcomes (such as return to work) emphasized in research on opioid therapy and the moment-to-moment experiences of pain patients. It also highlights the general issue that all of us – whether we suffer from chronic pain or not – must seek ways to balance out short-term rewards with longer-term ones. The point to emphasize here is that pain patients are by no means unique in their hypothesized valuation of short-term rewards. As an example of the trade-offs we all make between short and long-term rewards, consider ice cream consumption. Most of us would readily admit that ice cream is not good for one’s health, but we eat it anyway. Why? – because we experience momentary rewards as we consume them.
The fact that opioid therapy for chronic pain has few if any long-term benefits does not negate the possibility that patients might have good reasons for valuing it.
There is an urgent need to study the reasons why patients value opioids. Their perceptions might offer important insights into how a better balance between positive and negative effects might be achieved for CNCP patients receiving opioids.
If such research is done, it is almost certain to demonstrate that different patients have different reasons for valuing opioids. The specific hypothesis outlined above – that patients value opioids because of short-term relief following a dose – is likely to be valid for some patients, but certainly not for all of them.
The above hypothesis is amenable to empirical confirmation or disconfirmation and should be studied.
The hypothesized analogy between consuming opioids and consuming ice cream suggests a strategy that might be applicable to patients with chronic pain. Most people consume ice cream only occasionally – e.g., to celebrate some special occasion. This occasional use allows them to experience the rewards of ice cream consumption without suffering the ill effects of excessive consumption. By analogy, it’s possible that many CNCP patients can avoid the pitfalls of excessive opioid consumption by limiting their consumption to “special occasions” – that is, times when their pain is especially difficult to control.
- Agency Medical Directors’ Group. 2015 Opioid Dosing Guideline. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain - United States, 2022. MMWR Recomm Rep. 2022 Nov 4;71(3):1-95. doi: 10.15585/mmwr.rr7103a1. PMID: 36327391; PMCID: PMC9639433.
- Chou R, Hartung D, Turner J, Blazina I, Chan B, Levander X, McDonagh M, Selph S, Fu R, Pappas M. Opioid Treatments for Chronic Pain [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020 Apr. Report No.: 20-EHC011. PMID: 32338848.
- Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007 Mar 9;8:27. doi: 10.1186/1471-2474-8-27. PMID: 17349056; PMCID: PMC1829161.
- Häuser W, Thieme K, Turk DC. Guidelines on the management of fibromyalgia syndrome - a systematic review. Eur J Pain. 2010 Jan;14(1):5-10. doi: 10.1016/j.ejpain.2009.01.006. Epub 2009 Mar 4. PMID: 19264521.
- Vallerand A, Nowak L. Chronic opioid therapy for nonmalignant pain: the patient's perspective. Part I--life before and after opioid therapy. Pain Manag Nurs. 2009 Sep;10(3):165-72. doi: 10.1016/j.pmn.2009.03.007. PMID: 19706354.
- Vallerand A, Nowak L. Chronic opioid therapy for nonmalignant pain: the patient's perspective. Part II--Barriers to chronic opioid therapy. Pain Manag Nurs. 2010 Jun;11(2):126-31. doi: 10.1016/j.pmn.2009.03.006. Epub 2010 Apr 22. PMID: 20510843.
- Blake S, Ruel B, Seamark C, Seamark D. Experiences of patients requiring strong opioid drugs for chronic non-cancer pain: a patient-initiated study. Br J Gen Pract. 2007 Feb;57(535):101-8. PMID: 17263926; PMCID: PMC2034169.
- Hulen E, Saha S, Morasco BJ, Zeigler C, Mackey K, Edwards ST. Sources of Distress in Primary Care Opioid Management and the Role of a Controlled Substance Review Group: A Qualitative Study. Pain Med. 2018 Aug 1;19(8):1570-1577. doi: 10.1093/pm/pnx259. PMID: 29099982.
- Kennedy LC, Binswanger IA, Mueller SR, Levy C, Matlock DD, Calcaterra SL, Koester S, Frank JW. "Those Conversations in My Experience Don't Go Well": A Qualitative Study of Primary Care Provider Experiences Tapering Long-term Opioid Medications. Pain Med. 2018 Nov 1;19(11):2201-2211. doi: 10.1093/pm/pnx276. PMID: 29126138; PMCID: PMC6454789.
- Matthias MS, Johnson NL, Shields CG, Bair MJ, MacKie P, Huffman M, Alexander SC. "I'm Not Gonna Pull the Rug out From Under You": Patient-Provider Communication About Opioid Tapering. J Pain. 2017 Nov;18(11):1365-1373. doi: 10.1016/j.jpain.2017.06.008. Epub 2017 Jul 8. PMID: 28690000; PMCID: PMC6219456.
- Sullivan MD, Von Korff M, Banta-Green C, Merrill JO, Saunders K. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010 May;149(2):345-353. doi: 10.1016/j.pain.2010.02.037. Epub 2010 Mar 23. PMID: 20334974; PMCID: PMC3318978.
- Watson CP, Watt-Watson J, Chipman M. The long-term safety and efficacy of opioids: a survey of 84 selected patients with intractable chronic noncancer pain. Pain Res Manag. 2010 Jul-Aug;15(4):213-7. doi: 10.1155/2010/867201. PMID: 20808965; PMCID: PMC2935720.
- Robinson JP, Dansie EJ, Wilson HD, Rapp S, Turk DC. Attitudes and Beliefs of Working and Work-Disabled People with Chronic Pain Prescribed Long-Term Opioids. Pain Med. 2015 Jul;16(7):1311-24. doi: 10.1111/pme.12770. Epub 2015 Apr 30. PMID: 25929427.
- Ballantyne JC, Sullivan MD, Kolodny A. Opioid Dependence vs Addiction: A Distinction Without a Difference? Arch Intern Med. 2012 Sep 24;172(17):1342-3. doi: 10.1001/archinternmed.2012.3212. PMID: 22892799.
- Cofer CN, Appley MH. Motivation: Theory and research. New York: Wiley. 1964.
- Fields SA, Lange K, Ramos A, Thamotharan S, Rassu F. The relationship between stress and delay discounting: a meta-analytic review. Behav Pharmacol. 2014 Sep;25(5-6):434-44. doi: 10.1097/FBP.0000000000000044. PMID: 25089842.
- Rounds JS, Beck JG, Grant DM. Is the delay discounting paradigm useful in understanding social anxiety? Behav Res Ther. 2007 Apr;45(4):729-35. doi: 10.1016/j.brat.2006.06.007. Epub 2006 Aug 7. PMID: 16890909.
- Takahashi T, Oono H, Inoue T, Boku S, Kako Y, Kitaichi Y, Kusumi I, Masui T, Nakagawa S, Suzuki K, Tanaka T, Koyama T, Radford MH. Depressive patients are more impulsive and inconsistent in intertemporal choice behavior for monetary gain and loss than healthy subjects--an analysis based on Tsallis' statistics. Neuro Endocrinol Lett. 2008 Jun;29(3):351-8. PMID: 18580849.
- Mani A, Mullainathan S, Shafir E, Zhao J. Poverty impedes cognitive function. Science. 2013 Aug 30;341(6149):976-80. doi: 10.1126/science.1238041. PMID: 23990553.
- Kimura K, Izawa S, Sugaya N, Ogawa N, Yamada KC, Shirotsuki K, Mikami I, Hirata K, Nagano Y, Hasegawa T. The biological effects of acute psychosocial stress on delay discounting. Psychoneuroendocrinology. 2013 Oct;38(10):2300-8. doi: 10.1016/j.psyneuen.2013.04.019. Epub 2013 Jun 12. PMID: 23768971.
- Webster LR, Slevin KA, Narayana A, Earl CQ, Yang R. Fentanyl buccal tablet compared with immediate-release oxycodone for the management of breakthrough pain in opioid-tolerant patients with chronic cancer and noncancer pain: a randomized, double-blind, crossover study followed by a 12-week open-label phase to evaluate patient outcomes. Pain Med. 2013 Sep;14(9):1332-45. doi: 10.1111/pme.12184. Epub 2013 Jul 15. PMID: 23855816.
- Ashburn MA, Slevin KA, Messina J, Xie F. The efficacy and safety of fentanyl buccal tablet compared with immediate-release oxycodone for the management of breakthrough pain in opioid-tolerant patients with chronic pain. Anesth Analg. 2011 Mar;112(3):693-702. doi: 10.1213/ANE.0b013e318209d320. Epub 2011 Feb 8. PMID: 21304148.
- Davies A, Sitte T, Elsner F, Reale C, Espinosa J, Brooks D, Fallon M. Consistency of efficacy, patient acceptability, and nasal tolerability of fentanyl pectin nasal spray compared with immediate-release morphine sulfate in breakthrough cancer pain. J Pain Symptom Manage. 2011 Feb;41(2):358-66. doi: 10.1016/j.jpainsymman.2010.11.004. PMID: 21334555.
- Simpson DM, Messina J, Xie F, Hale M. Fentanyl buccal tablet for the relief of breakthrough pain in opioid-tolerant adult patients with chronic neuropathic pain: a multicenter, randomized, double-blind, placebo-controlled study. Clin Ther. 2007 Apr;29(4):588-601. doi: 10.1016/j.clinthera.2007.04.007. PMID: 17617282.
- Zacny JP, Drum M. Psychopharmacological effects of oxycodone in healthy volunteers: roles of alcohol-drinking status and sex. Drug Alcohol Depend. 2010 Mar 1;107(2-3):209-14. doi: 10.1016/j.drugalcdep.2009.10.012. Epub 2009 Nov 30. PMID: 19948383; PMCID: PMC2822039.
- Zacny JP, Paice JA, Coalson DW. Separate and combined psychopharmacological effects of alprazolam and oxycodone in healthy volunteers. Drug Alcohol Depend. 2012 Aug 1;124(3):274-82. doi: 10.1016/j.drugalcdep.2012.01.023. Epub 2012 Feb 25. PMID: 22365897; PMCID: PMC3568773.
- Zacny JP, Gutierrez S. Subjective, psychomotor, and physiological effects of oxycodone alone and in combination with ethanol in healthy volunteers. Psychopharmacology (Berl). 2011 Dec;218(3):471-81. doi: 10.1007/s00213-011-2349-6. Epub 2011 May 21. PMID: 21603891.
- Zacny JP, Gutierrez S, Kirulus K, McCracken SG. Psychopharmacological effects of oxycodone in volunteers with and without generalized anxiety disorder. Exp Clin Psychopharmacol. 2011 Apr;19(2):85-94. doi: 10.1037/a0022952. PMID: 21463065.
- Schmidt A, Denier N, Magon S, Radue EW, Huber CG, Riecher-Rossler A, Wiesbeck GA, Lang UE, Borgwardt S, Walter M. Increased functional connectivity in the resting-state basal ganglia network after acute heroin substitution. Transl Psychiatry. 2015 Mar 24;5(3):e533. doi: 10.1038/tp.2015.28. PMID: 25803496; PMCID: PMC4354356.
- Walter M, Denier N, Gerber H, Schmid O, Lanz C, Brenneisen R, Riecher-Rössler A, Wiesbeck GA, Scheffler K, Seifritz E, McGuire P, Fusar-Poli P, Borgwardt S. Orbitofrontal response to drug-related stimuli after heroin administration. Addict Biol. 2015 May;20(3):570-9. doi: 10.1111/adb.12145. Epub 2014 Apr 11. PMID: 24720731.
- Schmidt A, Borgwardt S, Gerber H, Wiesbeck GA, Schmid O, Riecher-Rössler A, Smieskova R, Lang UE, Walter M. Acute effects of heroin on negative emotional processing: relation of amygdala activity and stress-related responses. Biol Psychiatry. 2014 Aug 15;76(4):289-96. doi: 10.1016/j.biopsych.2013.10.019. Epub 2013 Nov 4. PMID: 24314348.
- Blum J, Gerber H, Gerhard U, Schmid O, Petitjean S, Riecher-Rössler A, Wiesbeck GA, Borgwardt SJ, Walter M. Acute effects of heroin on emotions in heroin-dependent patients. Am J Addict. 2013 Nov-Dec;22(6):598-604. doi: 10.1111/j.1521-0391.2013.12025.x. Epub 2013 Apr 11. PMID: 24131168.
- Greenwald MK, Hursh SR. Behavioral economic analysis of opioid consumption in heroin-dependent individuals: effects of unit price and pre-session drug supply. Drug Alcohol Depend. 2006 Oct 15;85(1):35-48. doi: 10.1016/j.drugalcdep.2006.03.007. Epub 2006 Apr 17. PMID: 16616994.
- Greenwald MK. Opioid craving and seeking behavior in physically dependent volunteers: effects of acute withdrawal and drug reinforcement opportunity. Exp Clin Psychopharmacol. 2005 Feb;13(1):3-14. doi: 10.1037/1064-1297.13.1.3. PMID: 15727498.
- Mackillop J, Murphy JG, Tidey JW, Kahler CW, Ray LA, Bickel WK. Latent structure of facets of alcohol reinforcement from a behavioral economic demand curve. Psychopharmacology (Berl). 2009 Mar;203(1):33-40. doi: 10.1007/s00213-008-1367-5. Epub 2008 Oct 17. PMID: 18925387; PMCID: PMC2774887.
- Johnson MW, Bickel WK. Replacing relative reinforcing efficacy with behavioral economic demand curves. J Exp Anal Behav. 2006 Jan;85(1):73-93. doi: 10.1901/jeab.2006.102-04. PMID: 16602377; PMCID: PMC1397796.
- Jacobs EA, Bickel WK. Modeling drug consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Exp Clin Psychopharmacol. 1999 Nov;7(4):412-26. doi: 10.1037//1064-1297.7.4.412. PMID: 10609976.